-
About
- Kyoto Prize
-
Research Grants
-
Social Contributions
- Events
- News
This website uses cookies to improve the user experience. If you continue on this website, you will provide your consent to our use of cookies.
About
Research Grants
Social Contributions

Assistant Professor, Graduate School of Engineering, Kyoto University *Profile is at the time of the award.
2022Inamori Research GrantsScience & Engineering
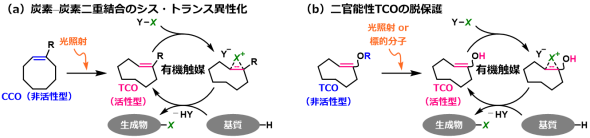
This study will develop unexploited organocatalysts to "observe" molecules, not to "synthesize" molecules.
Based on functions of carbon-carbon double bonds as a catalytic site, biocompatible visible-light gated catalysts were developed, and they enabled bromination of tyrosine. Moreover, biocompatible brominating reagents were developed. We are further trying to utilize them for the development of biomolecular labeling methods.
Science & Engineering