-
About
- Kyoto Prize
-
Research Grants
-
Social Contributions
- Events
- News
This website uses cookies to improve the user experience. If you continue on this website, you will provide your consent to our use of cookies.
About
Research Grants
Social Contributions

Lecturer, Graduate School of Pharmaceutical Sciences, The University of Tokyo *Profile is at the time of the award.
2021Inamori Research GrantsScience & Engineering
This project that we have been working on with students for several years is approaching the first goal. With the support of this grant, we hope to further accelerate and improve the quality of research.
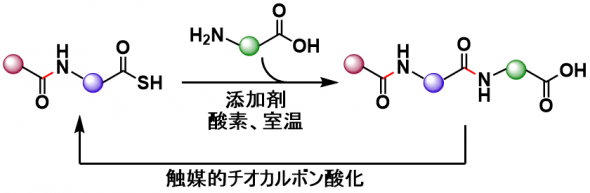
N-to-C peptide synthesis that suppresses epimerization and improves the reaction efficiency by using C-terminal thiocarboxylic acid conversion – main-chain unprotected amino acid elongation was optimzed.
For this purpose, a new additive, HOPO(Phy), which can be easily removed, recovered, and reused, was identified. The optimized conditions proceeds in high yield and low epimerization revel. Convergent synthesis of bioactive nonapeptides was possible using this process.
Science & Engineering